

The infusion device is mainly composed of a jacket, a c […]
The infusion device is mainly composed of a jacket, a core rod, a piston, a positioning ring, and a protective cap. It uses imported raw materials and is produced by an injection molding process. The infusion device is mainly used to contain medicines for injection. The main sterilization method used for this product is ethylene oxide sterilization or cobalt 60 sterilization. It can effectively ensure that the product is sterile before use. The scale value can be printed on the push rod to ensure the accuracy of medication.
Precautions for storage of the perfusion device:
1. Ensure that the syringe will not be exposed to the sun for a long time.
2. Place it in a place that is not easy for children to touch.
3. Do not place the plastic syringe in a humid and cold environment.
4. Prevent the syringe from falling from a high place.
5. Fasten the protective cap of the prefilled syringe containing the liquid medicine to prevent the liquid medicine from overflowing.
6. Avoid direct contact with other chemical solvents.
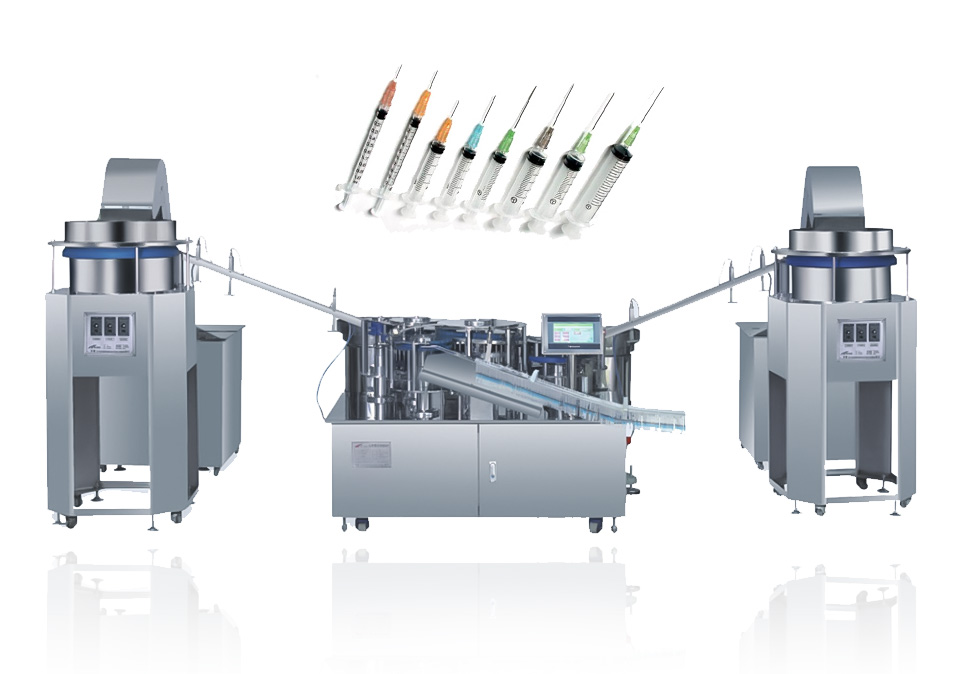
Pre-filled syringes are mainly made of glass and plastic materials. Plastic pre-filled syringes are gradually replacing glass syringes. They mainly have lower cost, higher safety than glass, and are not easy to break. They are relatively high-quality products. The production process itself is relatively advanced. Generally speaking, the injection molding process is used to produce prefilled syringes. The products produced by the injection molding process are molded at one time, with no leftovers, accurate product size and uniform weight.
Prefilled syringes have high requirements on the product's tightness. When selecting the syringe, the tightness of the product should be tested. Fill the syringe with half of the water, insert the piston into the syringe, remove the protective cap to remove residual air, and then use the protective cover. If the cap is blocked, apply an axial pressure of 30N on the push rod rubber plug inside the syringe through the push rod, and keep it for thirty seconds. There should be no leakage at the seal of the protective cap and the seal of the piston.
Because of direct contact with drugs, the sterility of prefilled syringes must be guaranteed. Generally speaking, they should be produced in a clean workshop that meets national standards. In addition, to ensure sterility, if the manufacturer has higher requirements for sterility, Additional ethylene oxide sterilization or cobalt 60 irradiation sterilization is performed.
https://www.syringeassemblymachines.com/