

The prefilled syringe needs to meet the following three […]
The prefilled syringe needs to meet the following three requirements:
1. In order to ensure that the medicine will not be damp or deteriorating during the validity period, the prefilled syringe has good sealing and barrier properties, and can prevent the influence of light, heat, water vapor, oxygen, etc. on the medicine.
2. The inner wall of the sleeve of the prefilled syringe is in direct contact with the drug, and the bottle-making material must meet the requirements of the drug packaging to ensure the safety of the drug.
3. The size and structure of the pre-filled syringe should be adapted to the requirements of the filling machine of pharmaceutical companies and the requirements of the high-speed automatic filling machine. The prefilled syringe has the advantages of light weight, high strength, not easy to break, easy to transport, good sealing performance, moisture-proof, hygienic, and meets the special requirements of pharmaceutical packaging. It can be directly used for pharmaceutical packaging without washing or drying. An excellent medicinal packaging container.
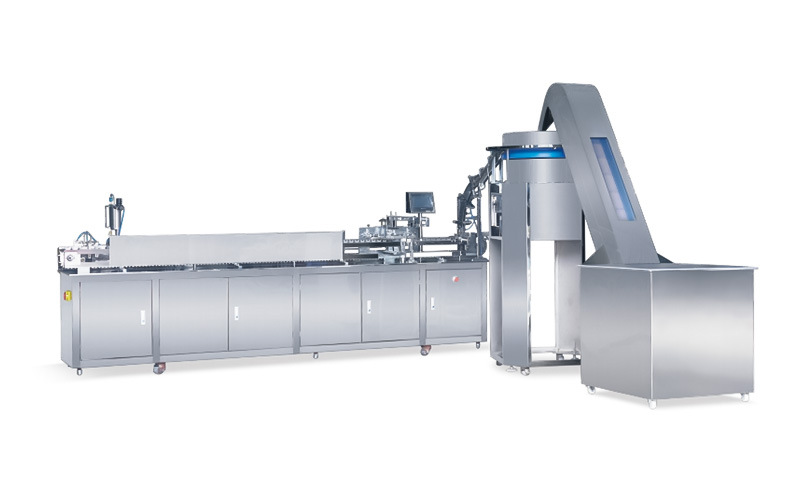
The infusion device is composed of a needle tube, a cap, a push rod or a piston. The sterility of the perfusion device and the detection of abnormal toxicity are its two important indicators.
Test method for sterility of perfusion device:
(After sterilization) Take an appropriate amount of this product, add 1/2 of the labeled volume of sodium chloride injection, cover the piston and cap, shake for 1 minute, and combine the extracts. Take the extract and inoculate 2 small tubes of thioglycolate fluid medium (TG) (9ml per tube), 0.2ml each; one is cultured at 35~37℃, and the other is cultured at 23~25℃. Another small TSB tube (7ml per tube) was used to inoculate 0.2ml, cultured at 23~25℃, and checked according to the sterility inspection method, which should meet the requirements.
Abnormal toxicity detection of perfusion device:
Take a few of this product, clean the syringe with water, cut it into pieces, take 500cm2 (in terms of internal surface area), add 50ml of sodium chloride injection, put it in an autoclave at 110°C for 30 minutes, take it out, cool it for later use, The same batch of sodium chloride injection was blanked and injected intravenously, and the inspection should be in accordance with the regulations.
https://www.syringeassemblymachines.com/