

The infusion device is composed of a needle tube, a cap […]
The infusion device is composed of a needle tube, a cap, a push rod or a piston. The sterility of the perfusion device and the detection of abnormal toxicity are its two important indicators.
Test method for sterility of perfusion device:
(After sterilization) Take an appropriate amount of this product, add 1/2 of the labeled sodium chloride injection, cover the piston and cap, shake for 1 minute, and combine the extracts. Take the extract to inoculate 2 small tubes of thioglycolate fluid medium (TG) (9ml per tube), 0.2ml each; one is incubated at 35~37℃, and the other is incubated at 23~25℃. Another small TSB tube (7ml per tube) was used to inoculate 0.2ml, cultured at 23~25℃, and checked according to the sterility inspection method.
Abnormal toxicity detection of perfusion device:
Take a few of this product, clean the syringe with water, cut it into pieces, take 500cm2 (in terms of inner surface area), add 50ml of sodium chloride injection, put it in an autoclave at 110°C for 30 minutes, take it out, cool it for later use, The same batch of sodium chloride injection was blanked and injected intravenously, and checked in accordance with the law.
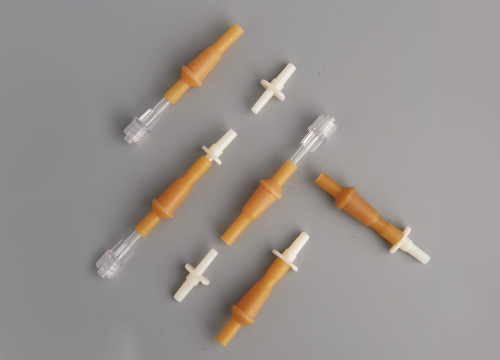
The infusion device component is composed of a needle tube, a cap, a push rod or a piston, and each plastic component is produced by injection molding. The infusion device is generally sterilized with ethylene oxide. Ethylene oxide will produce residue after sterilization, and the residual amount of ethylene oxide should be ≤1µg/ml before the product can be used.
Detection method of ethylene oxide residue in the perfusion device: Sample preparation: Take this product and remove the package, suck in distilled water of the marked volume (V), and equilibrate at 37℃±1℃ for 1 hour.
Control stock solution preparation: Take a 50ml volumetric flask which is dry outside, add 30ml water, add a stopper, and weigh to the nearest 0.1mg. Use a syringe to inject 0.6ml of ethylene oxide reference substance (purity greater than 99.7%), shake it gently, cover the bottle stopper, and weigh. The difference between the two weighings is the weight of ethylene oxide. Add water to the mark and dilute this solution to 1g/L as a control stock solution. Dilute it into a solution with an ethylene oxide content of 1 µg/ml as a control solution.
Take 5ml of each of the control solution and the sample and place them in the top empty bottle, and place them at 60°C±1°C for 20 minutes. Inject 1ml of the overhead gas into the gas chromatograph, record the chromatogram, and measure the peak area of the reference substance and the sample. The peak area of the control solution should be greater than the peak area of the sample.
https://www.syringeassemblymachines.com/