

The prefilled syringe has a simple structure and is mai […]
The prefilled syringe has a simple structure and is mainly composed of a tube sleeve, a push rod, a protective cap, and a piston. The push rod can be printed with scales, which can effectively ensure the accuracy of medication. The prefilled syringe is sterile and free of cross-infection. It can be mainly used in packaging of medicines and nutritional creams.
Requirements for the use of prefilled syringes:
1. The prefilled syringe is a sterile product and should be stored immediately after filling.
2. This product is only available for one-time filling, it is destroyed after use, and repeated use is prohibited.
3. It is forbidden to use the syringe with high concentration chemical products at the same time.
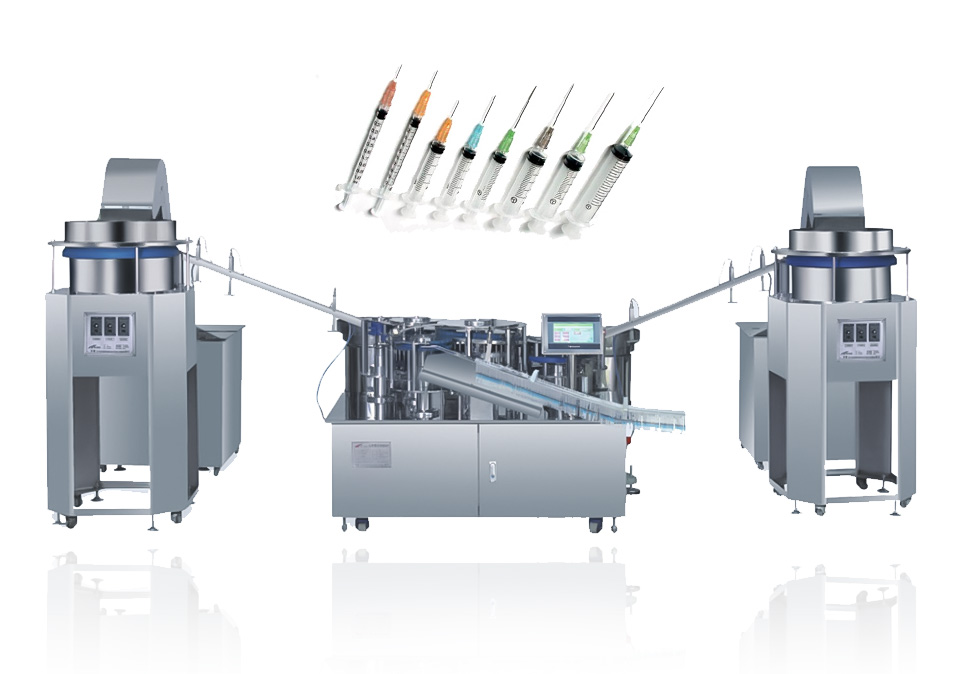
The prefilled syringe is produced by injection molding process with high mold precision. The workshop purification level is D level, and the main requirements are proper tightness, good sealing and sterility.
The infusion device is composed of a needle tube, a cap, a push rod or a piston. Generally, it can be used for udder injection of dairy cows. The sterility and abnormal toxicity of the perfusion device are two important indicators.
Test method for sterility of perfusion device:
(After sterilization) Take an appropriate amount of this product, add 1/2 of the labeled volume of sodium chloride injection, cover the piston and cap, shake for 1 minute, and combine the extracts. Take the extract and inoculate 2 small tubes of thioglycolate fluid medium (TG) (9ml per tube), 0.2ml each; one is cultured at 35~37℃, and the other is cultured at 23~25℃. Another small TSB tube (7ml per tube) was used to inoculate 0.2ml, and cultured at 23~25℃, according to the sterility inspection method (inspection should meet the requirements.
Abnormal toxicity detection of perfusion device:
Take a few of this product, clean the syringe with water, cut it into pieces, take 500cm2 (in terms of internal surface area), add 50ml of sodium chloride injection, put it in an autoclave at 110°C for 30 minutes, take it out, cool it for later use, The same batch of sodium chloride injection was blanked and injected intravenously, and the inspection should be in accordance with the regulations.
https://www.syringeassemblymachines.com/